IntegriCulture Publishes Research on Breakthrough Technology to Accelerate Commercialization of Cellular Agriculture — Paper on Safe, Low-Cost, Food-Grade Reagents Developed via a “Food Regulation Pre-Check” Framework Appears on ChemRxiv —
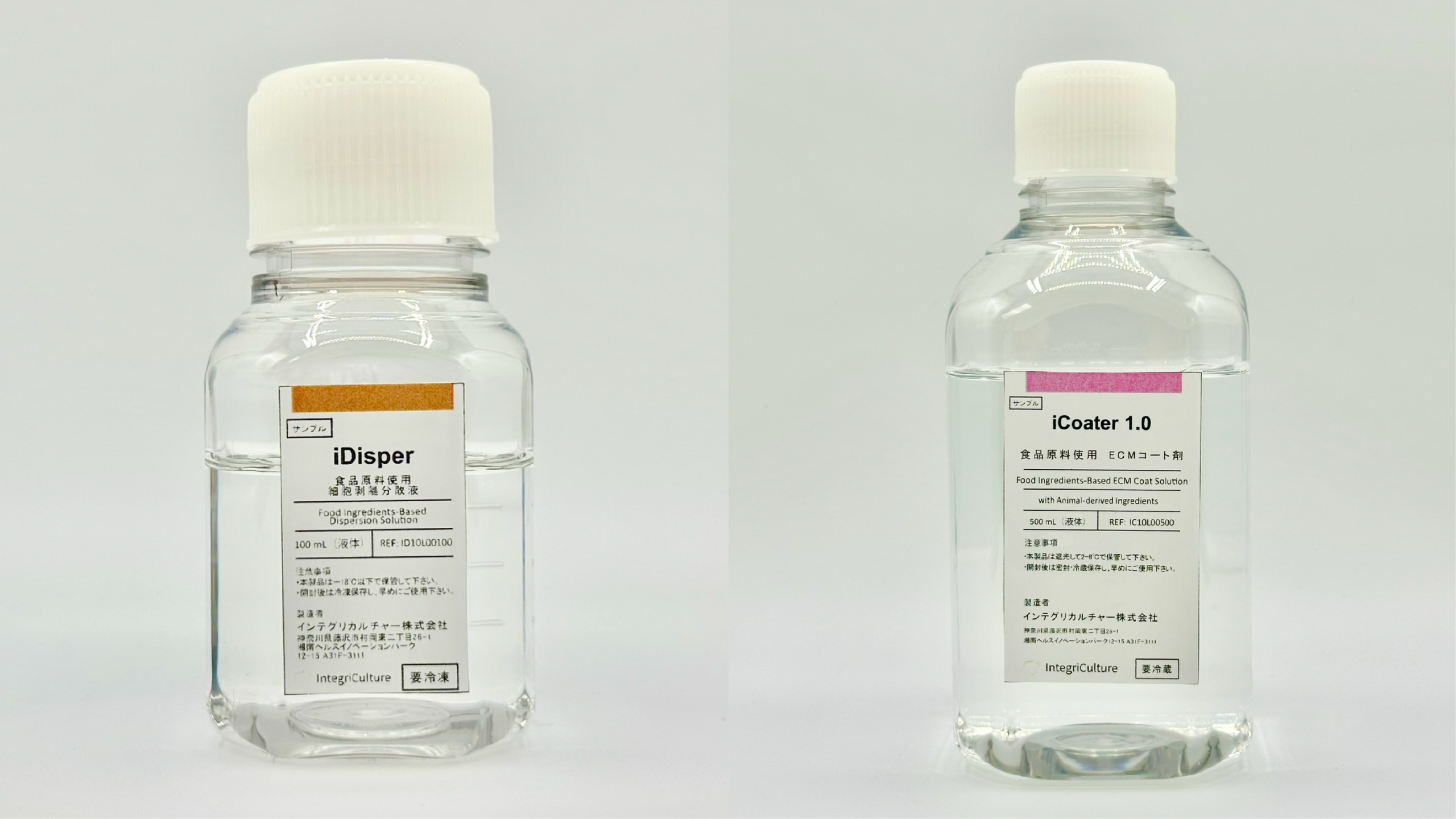
TOKYO, JAPAN, September 19, 2025 – IntegriCulture Inc., a Japanese company pioneering cellular agriculture, today announced the development of a novel framework and two new food-grade cell culture agents, “iDisper” and “iCoater,” designed to overcome the major barriers to the commercialization of cellular agriculture: high costs and safety risks.
The company also announced that two manuscripts detailing these findings and their scientific validation was published today, September 2025, on the preprint server ChemRxiv.
This innovative approach, now supported by public scientific data, transforms unknown risks into manageable ones, paving a clear path to market within existing food safety regulations and significantly advancing the widespread availability of safe and affordable cell-based food.
Background: The Commercialization Hurdles for Cell-based Food The commercial viability of cell-based foods has been hindered by the high cost of expensive pharmaceutical-grade reagents and the safety risks associated with animal-derived materials, such as porcine trypsin, which carry potential risks of viral contamination and allergens. To fundamentally address these issues, IntegriCulture has reimagined the entire raw material development process.
A New Development Framework: “Food Regulation Pre-Check” Unlike conventional development processes that first evaluate functionality, IntegriCulture has introduced a new framework: the “Food Regulation Pre–Check.” This approach begins by screening potential materials for their compliance with food regulations. This ensures that any risks that could impede commercialization are eliminated at the earliest stage, enabling safer and more efficient product development.
Two New Food-Grade Reagents
1. iDisper: An Animal-Free Cell Dissociation Agent Used to detach cultured cells, iDisper is made entirely from food additives: the plant-derived enzyme “papain” and “trisodium citrate.” By completely avoiding animal-derived components, it eliminates the risk of viral contamination. Performance tests have confirmed that iDisper achieves cell detachment efficacy and viability comparable to commercial reagents, while maintaining stable performance for eight months at -20°C.
2. iCoater: A Ready-to-Use Cell Culture Coating Agent Used to coat culture surfaces, iCoater is made from food-grade gelatin and is applied to standard food manufacturing with heat-sterilized. This method enables the production of a safe and stable product at low cost by leveraging existing food-grade infrastructure, removing the reliance on expensive pharmaceutical-grade facilities.
Significance: A Paradigm Shift From Unknown Risks to Manageable Risks
This framework fundamentally shifts the risk paradigm. It transforms “hidden, unknown risks,” such as viral contamination from porcine materials, into “manageable, visible risks” that can be addressed through familiar measures like allergen labeling. This approach enables a clear commercialization pathway within existing food safety systems, helping to build consumer trust.
This announcement presents a comprehensive blueprint—from foundational material development to social implementation—that will accelerate the adoption of safe and affordable cellular foods.
Comment from Yuki Hanyu, CEO of IntegriCulture Inc.: “To make cellular foods an everyday option that consumers can enjoy with complete peace of mind, we must overcome the barriers of safety and cost with scientific evidence. We are proud to share our novel ‘Food Regulation Pre-Check’ framework and its successful results with the world in the form of a transparent, scientific paper. We are confident that this work will contribute to the sustainable growth and integrity of the entire cellular agriculture industry.”
Publication Details of “iDisper”
- Title: Dissociation Alternative Product Developed via a Food Regulatory Pre-Check Framework
- Authors: Hiroaki Hatano, Misaki Sawada ,Misaki Kitsukawa ,Azusa Hayakawa ,Hiroaki Kondo ,Ikko Kawashima
- Server: ChemRxiv
- Date of Publication: September 24, 2025
- DOI (Digital Object Identifier):10.26434/chemrxiv-2025-g3jdp
Publication Details of “iCoater”
- Title:A Food-Grade Ready-to-Use Cell Culture Coating Agent Developed via Food Manufacturing: A Dual Technical and Regulatory Approach
- Authors: Hiroaki Hatano, Misaki Sawada ,Misaki Kitsukawa ,Azusa Hayakawa ,Hiroaki Kondo ,Ikko Kawashima
- Server: ChemRxiv
- Date of Publication: September 19, 2025
- DOI (Digital Object Identifier):10.26434/chemrxiv-2025-9gfsq
Media Contact:
IntegriCulture Inc.
Email: info@integriculture.com
